Research Overview
Our department is committed to developing treatments for neurodegenerative diseases, in particular Alzheimer’s disease, so that people can live a good and healthy life.

Professor
Department of Brain and Neurodegenerative Disease Research
Minako Hoshi, Ph.D.
Our department is committed to developing treatments for neurodegenerative diseases, in particular Alzheimer’s disease, so that people can live a good and healthy life.

For humans, “memory” is not just a “record.” We develop the ability to visualize the future that we want from our memories, to select what is needed to make it happen and to change the neuronal network according to our experiences. This is the essential difference between the brain and a computer. When a person develops Alzheimer's disease, which causes dementia, the neuronal network that has been developed over many years is slowly damaged. Eventually, neurons are completely lost. This disease gradually robs you of who you are and ultimately leaves you unable to do anything for yourself.
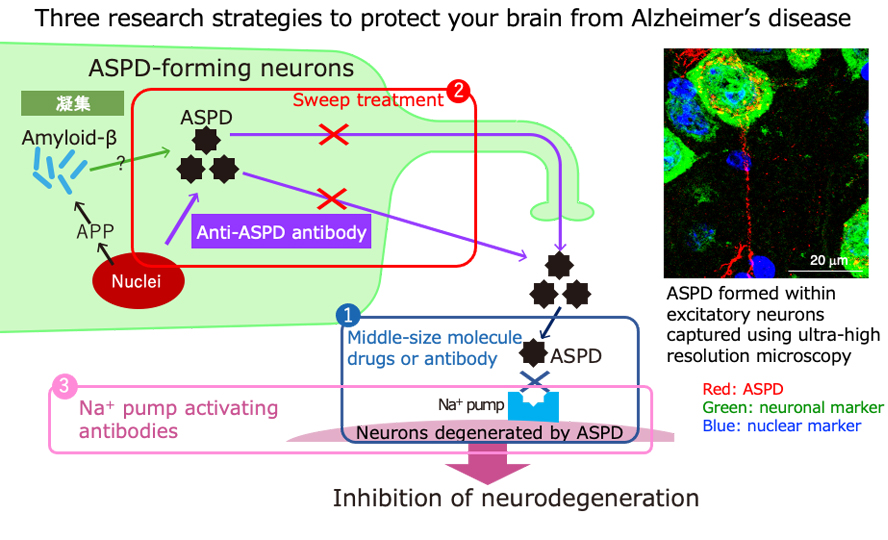
Recently, it has been reported that antibodies against a small protein called amyloid-β protein (Aβ) can ameliorate Alzheimer's disease to some extent, giving us a ray of hope for an effective treatment. Aβ is an important protein that protects neurons in healthy brains, but it has a soft string-like structure that can take various forms. Multiple studies have shown that when Aβ assembles into abnormal shapes, the resulting Aβ assemblies become toxic. There are many forms of Aβ assemblies that exert different toxicity depending on their form. Thus, little is yet known about why antibodies that have been approved as drugs are effective.
We have studied the various forms of Aβ assemblies and their roles for many years. As a result, we discovered that when about 30 molecules of Aβ aggregate together, they form an ordered sphere with spines like a sea urchin. These spheres have a strong toxicity and destroy neurons and neuronal networks. We named these sea urchin-like toxic spheres “amylospheroids” (ASPD). We further discovered that the spines on ASPD bind to the sodium pump, which is essential for the function and survival of neurons, leading to the death of the neurons. Thus, for the first time, one of the causes of Alzheimer's disease was identified. Once this was established, several treatment strategies could be considered, including blocking ASPD binding to the sodium pump or removing ASPD themselves. We are currently working along these lines, collaborating with researchers from other fields. Our ultimate aim is to prevent the disease so that people need not fear dementia and can live a good and healthy life as they age.
Our main current ongoing projects are: