Overview
A cure for Alzheimer's disease has not yet been established because the mechanism of its onset has not been properly understood. Dr Hoshi, Professor of the Department for Brain and Neurodegenerative Disease Research, Foundation for Biomedical Research and Innovation at Kobe, and colleagues have proved that a unique toxic structure termed amylospheroid inhibits the action of the neuron-specific sodium pump, which is essential for neuronal activity, and causes neuronal death, leading to Alzheimer's disease. They have succeeded in developing an antibody that specifically recognizes a structure of amylospheroid and a drug candidate that blocks amylospheroid from binding to the sodium pump. This time, using the antibody, we devised a new means to autolyze only toxic structures in neurons (cleaning using the mechanism inherent in the living cells), and proposed multifaceted treatment based on a correct understanding of the onset mechanism. Since the above treatment strategy requires the accumulation of original technology, it can be implemented only by this research institute. When this proposal was published in the February issue of the prestigious British Journal of Pharmacology, it was recognized as an epoch-making proposal linked to the fundamental treatment of Alzheimer's disease, and was adopted on the cover page, and received extremely high praise.
1. Background
The number of patients with Alzheimer's disease (AD) is estimated to reach 50 million worldwide in 2018 and exceed 150 million in 2025, and the emergence of radical therapeutic agents is awaited. Prof Hoshi has been working to elucidate the molecular mechanism of neuronal death in the AD brain since 1997 and discovered the toxic structure termed amylospheroids (ASPD) *1 that is thought to trigger neuronal death and reported it in the Proceedings of the National Academy of Sciences of the United States of America in 2003. In 2015, Prof Hoshi has proved that ASPD binds to a neuron-specific sodium pump*2 and inhibits its pump activity to induce neuronal cell death, which was reported in the Proceedings of the National Academy of Sciences of the United States of America. Sodium pumps are essential for the survival and function of neurons and were not expected to be involved in disease prior to the reports of Hoshi et al. However, following the discovery of Hoshi et al., similar reports of Parkinson's disease and amyotrophic lateral sclerosis followed (Shrivastava et al. EMBOJ., 2015; Ruegsegger et al. Acta Neuropathol. 2016). Since then, the relationship between the sodium pump and neurodegenerative diseases is drawing attention.
2. Research method / results
Amyloid β is the most promising trigger for the onset of AD, but the path to therapeutic drug development is extremely difficult. One of the reasons is that amyloid β itself, before aggregation, has a neuroprotective effect, and the other reason is that the stage where toxic aggregates of amyloid β are detected in the brain has already advanced to the stage of disease, which is difficult to be cured. Therefore, it is important to remove toxic aggregates as early as possible and selectively. From that point of view, Prof Hoshi newly discussed the development of AD treatment methods targeting amyloid β, and what kind of treatment strategies are possible now, focusing on their research results.
One of the specific strategies proposed is to mask the surface of the amyloid β assembly with a medium-molecular-weight drug*3, which is currently attracting attention as next-generation drug discovery, to prevent interaction with the sodium pump. The second is to activate the sodium pump itself with a small molecule drug. The third is to dissect the amyloid β assembly formation pathway in neurons and to develop ultra-early medical treatment by removing the assembly itself inside of neurons before it is secreted.
The above three points are the projects that Prof Hoshi’s department is currently working on. In particular, the final strategy is currently underway because the recent research findings have convinced Prof Hoshi that early and selective removal of toxic aggregates is feasible. This is a completely new initiative that was made possible for the first time because they have tools (antibodies) and analysis systems that selectively capture toxic aggregates.
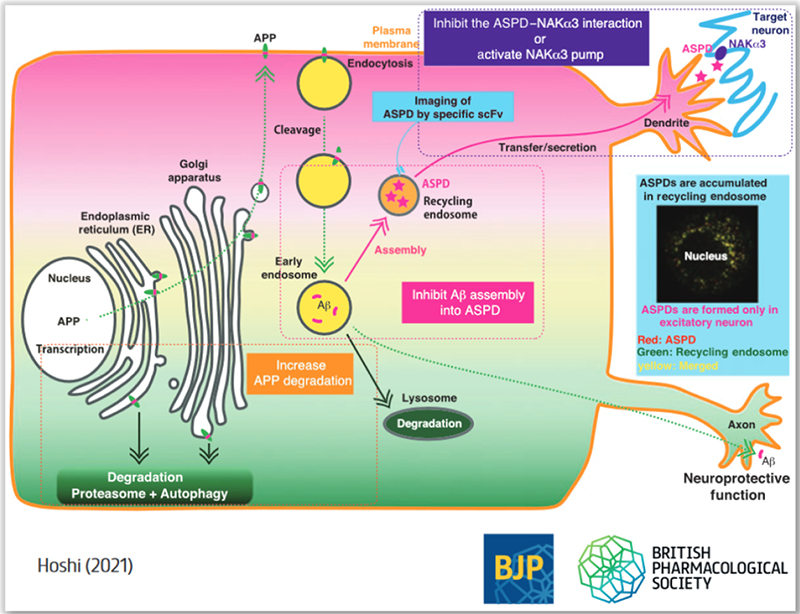
The concept was highly evaluated by Professor Amrita Ahluwalia, Editor-in-Chief, and Professor Giuseppe Cirino, Review & Themed Issue Senior Editor, and was adopted as the BJP cover of the February 2021 issue of the Journal of the British Pharmacological Society. (Figure above).
3. Spillover effect
As for the development of AD therapeutic agents, the situation remains unclear. There is no doubt that amyloid β is the earliest detectable trigger at this time, but there are still many unclear points such as why aggregation occurs in the first place, and there is still much to be done in basic research. This time, Prof Hoshi proposed a treatment strategy that is currently possible based on her research results. Since it has been shown that inhibition of the neuron-specific sodium pump activity by aggregates may also be the cause of Parkinson's disease, the therapeutic strategy proposed here has a spillover effect far beyond Alzheimer's disease. All the proposed treatment strategies require a high degree of technical accumulation and can only be carried out by this research institute.
4. Future Plans
To leave a future for the next generation where people can spend their lives with a smile to the end, this research institute is advancing the above three strategies together with a company that wants to challenge the development of treatment methods with us. Please contact the public relations for joint research.
Title and Authors
Journal: Br. J. Pharmacol.
Title: Multi‐angle development of therapeutic methods for Alzheimer's disease
Author: Minako Hoshi1
1Department of Brain and Neurodegenerative Disease Research, Institute of Biomedical Research and
Innovation, Foundation for Biomedical Research and Innovation at Kobe, Kobe, Japan.
DOI: 10.1111/bph.15174
Author/Contact
Minako Hoshi/PhD

Professor and Director,
Department of Brain and Neurodegenerative Disease Research,
Institute of Biomedical Research and Innovation,
Foundation for Biomedical Research and Innovation at Kobe
URL: https://www.fbri-kobe.org/english/laboratory/research3
CONTACT
Glossary
*1 amylospheroids
Spherical assemblies with strong neurotoxicity formed in neurons of Alzheimer's disease patients found by Hoshi et al. Approximately 30 amyloid β aggregate to form a specific spherical structure, which binds to the sodium pump on the neuronal surface and inhibits pump activity, thereby exerting toxicity.
*2 Sodium pump
It exists on the cell membrane of almost all animal cells, and by using the chemical energy of ATP, it pumps 3 sodium ions from the inside to the outside per ATP molecule against the concentration gradient and 2 potassium ions from the outside to the inside. The difference in the concentration of sodium produced by this pump protein makes it possible for neurons to be excited and plays an important role as the basis of the vital activities of all cells including neurons. In recent years, Hoshi et al. and other researchers have found that abnormalities in neuron-specific sodium pumps are associated with neuropathy in Alzheimer's disease and Parkinson's disease, and are drawing new attention to their relationship with neurodegenerative diseases.
*3 Medium molecule drug
A drug with a molecular weight of about 500 to 2000, which is positioned between a low molecular weight drug with a small molecular weight and an antibody-drug with a large molecular weight. It is expected to a next-generation drug discovery that combines the advantages of small molecule drugs and antibody drugs.
Oral administration is also possible, and since it can be chemically synthesized, the manufacturing cost can be kept low. By using medium molecules, it will be possible to approach not only the targets targeted by antibody drugs, but also intracellular molecules that require high specificity, which cannot be targeted by small molecules and antibodies, and intracellular protein / protein interactions and protein / nucleic acid interactions.