Let’s take a look at KBIC’s achievement record!

KBIC’s Beginning - First Time Ever for Japan
When KBIC was first established in 1998, Japan had never experienced an initiative of this kind, let alone one of this scale and magnitude. As such, KBIC was an unprecedented vision for which there was no map or blueprint. Yet, ultimately, it has become an initiative and model for the next generation of science and industry.
Immediately following KBIC’s establishment, RIKEN, Japan's largest comprehensive research institution renowned for its high-quality research in a diverse range of scientific disciplines, opened its doors at KBIC in 2000, simultaneously with the establishment of the Foundation for Biomedical Research and Innovation(FBRI) as the core organization supporting KBIC, which is currently headed by Dr. Shuh Narumiya.

Record of Continuous Results. In a little over 20 years, the presence of RIKEN and the creation of FBRI, in turn, has led to the concentration of about 370 companies, organizations and research institutions as well as a number of highly specialized hospitals at KBIC, which, individually and collectively, have generated myriad results and accumulated many groundbreaking achievements through joint projects and other forms of innovative collaboration based on trans-sector bridging.
Fast forward a few years to 2014 when RIKEN, FBRI and hospitals at KBIC teamed up to achieve the world’s first surgical procedure to successfully transplant iPS cells into the eye of a human patient with macronuclear degeneration, resulting in the new field of cell therapy.
As described above, with more than 20 years of achievements and a record of innovative collaboration, KBIC has demonstrated that its unique model of collaboration among the industrial, governmental, academic, and healthcare sectors continuously generates results on a steady basis, not only in the area of regenerative medicine but in the healthcare sector as a whole.
Please read a bit further for more of KBIC’s achievements.
1. Nobel Laureate - Dr.Tasuku Honjo/FBRI

Dr. Tasuku Honjo, an Honorary President of FBRI, was the recipient of the 2018 Nobel Prize in Physiology or Medicine for the discovery of a protein that has proved effective in cancer treatment. Dr. Honjo serves as Deputy Director-General and Distinguished Professor of the Kyoto University Institute for Advanced Study.
Established by Kobe City, FBRI has been an integral part of KBIC and has played an active role in KBIC’s development and activities since KBIC’s establishment. This discovery of Dr. Honjo has revolutionized the treatment of cancer. In addition, the Institute of Biomedical Research and Innovation (IBRI), one of FBRI’s research institutes, has published scientific articles about their various recent cutting-edge research results.
Representative scientific articles recently published from IBRI can be found at https://www.fbri-kobe.org/english/laboratory/
2. Location of first clinical experiments for iPS cells
At KBIC, the world’s first-ever regenerative medicine clinical trials using induced pluripotent cells (iPS cells) were carried out. In August 2013, with the cooperation and support of the Kobe City Medical Center General Hospital, RIKEN and the Institute of Biomedical Research and Innovation (IBRI) began clinical research on patients with wet age-related macular degeneration, an intractable disease of the retina, based on iPS cell-derived retinal sheet transplantation ("autologous transplantation"), and subsequently performed the world's first iPS transplant surgery in September 2014.
In addition, in June 2016, the Center for IPS Cell Research and Application (CiRA) of Kyoto University, RIKEN, Kobe City Medical Center General Hospital, and Osaka University Hospital launched a new clinical research project involving allogeneic transplantation, which involves transplanting retinal cells derived from the iPS cells of another person, with the aim of early commercialization. More specifically, this entailed generating retinal cells from a patient’s fibroblasts that were then transplanted into the eyes of a different patient. The first transplant for this project was successfully carried out in March 2017.This was a collaborative project involving four of KBIC’s research institutes, chief of which was the Kobe City Medical Center General Hospital.
The Kobe Eye Center was opened in December 2017 to further support the early commercialization of regenerative eye medicine using iPS cells. Currently, regenerative medicine using iPS cells is also used in clinical trials for Parkinson’s disease and spinal cord injuries.
3. Achievements of TRI

As Japan’s first academic data and statistical analysis center, TRI is committed exclusively to transforming basic medical research into useful clinical applications. Toward this end, TRI provides comprehensive support programs for medical researchers in all phases of clinical study ranging from research planning to data analysis both in Japan and overseas.
TRI has played a major role in the following major medical breakthroughs, among others:
- Active involvement on the development of regenerative medicine: Treatment involving the regeneration of nerves and eardrums have been approved and are being provided in clinical practices. Regeneration of blood vessels, myocardium, corneas, and bone are underin clinical trials, for the purpose of aiming for PMDA and other approvals in Japan and elsewhere.
- Cancer initiatives: TRI has supported large-scale clinical trials on prostate and colorectal cancers, showing high overall survival rates for prostate and stage II colorectal cancer.
- Challenges for dementia: Our cohort study with China shows that intervention from Mild Cognitive Impairment(MCI) is required to prevent the progression to Alzheimer's disease(AD), and TRI is supporting the unique trial for MCI.
- Elder care: TRI has developed a novel dementia scale for AD, as well as harnessing regenerative medicine to improve healthcare for the elderly.
- Global collaborations: TRI is actively engaged in collaborations with universities and university-affiliated research institutes in Taiwan, China, Korea, Singapore, the EUs, and the US to tackle critical challenges involved in the fight against disease control.
For more information, click here » TRI
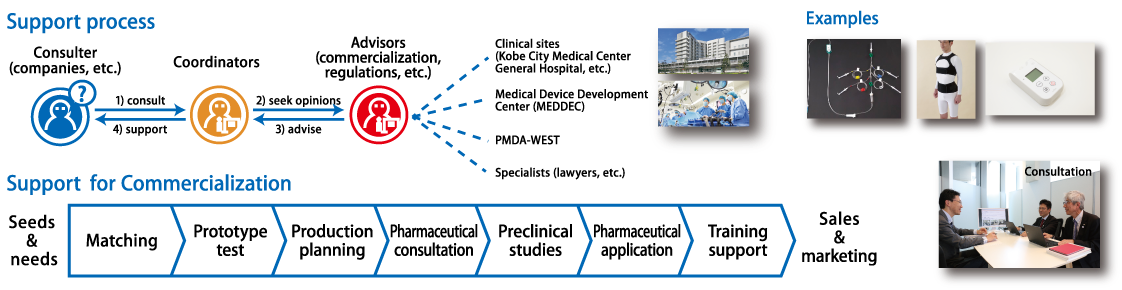
4. Commercialization of new medical devices
The coordinators on the medical device team at FBRI’s Center for Cluster Development (CCD) have successfully launched 56+ products into the market (as of 2022). Below are two representative products which are recognized as commercially viable products due to their innovation.
- ANTILEAK: No leaking infusion bag for anticancer drug or other exposure drugs to protect medical staff from exposure.
- SmartCuff©: Handy, battery-typed, automatic controlled pressure controller
These and other products are the result of our consulting and supporting activities for business planning, regulatory science, market research, patent issues, technical designing and other subjects related to materialize innovative ideas by turning them into products. This service has been provided to companies, universities, researchers and hospitals located in KBIC free of charge and is the feature that differentiates KBIC from other industrial clusters in Japan and elsewhere.
In addition to this face-to-face service, KBIC provides a training facility called MEDDEC (Kobe Medical Device Develop Center) to innovators. MEDDEC can be used for evaluation of newly designed mockup products in a real surgical environment, and has no user limitations if they have a passion to make a success in Kobe. This open innovation philosophy attracts transnational or international innovators and has led to the successful launch of 56 products.
5. Location of the Supercomputer used for drug discovery
Shared use at KBIC of the supercomputer, which was originally jointly developed by RIKEN and Fujitsu, started in 2012. The supercomputer "Fugaku", as a successor to the K computer which was in operation until 2019, has been in full-scale operation since March 2021 and widely used in such as simulations in areas of infectious disease control and extreme weather prediction.
The capability of the supercomputer to process massive volumes of big data has changed the face of drug discovery and can lead to significantly reduced time and expenses as well as the increased probability of new drug discovery. Click for more details. (URL:https://www.fbri-kobe.org/kbic/english/benefit/)
"Fugaku" is another name for "Mt. Fuji". Its height represents the high performance of the supercomputer Fugaku, and the vastness of its foot means the expansion of the users (of Fugaku).
Fugaku continues to maintain its top-class performance of the supercomputing world.
[Ext.](URL:https://www.r-ccs.riken.jp/en/outreach/topics/20221117-1/)
6. Proven track record for startup companies
To date, the projects and innovative collaboration at KBIC have resulted in numerous startup companies in a wide variety of healthcare areas. For more details, click here. » Startups & Investors
7. WHO collaboration-“Kobe Model” for dementia
FBRI, Kobe University, Kobe Gakuin University (all a part of KBIC) and WHO (Kobe Center) launched a joint project to develop an integrated system for early detection, diagnosis and intervention of dementia, known as the "Kobe Model". The evidence gathered from the study of 80,000 Kobe residents is expected to be of great help in addressing the challenges of dementia worldwide. For information, click here.» WHO Research Projects
8. Involvement with the 500 Kobe Accelerator Program

KBIC’s support and fostering in the healthcare area takes many forms. One of them is its involvement in 2019 together with 500 Startups, a Silicon Valley early-stage venture fund, to target new health/technology businesses in the 500 Kobe Accelerator Program which provides mentoring and business support to startups and early-stage companies. Participants in the program come from Japan and from overseas.