The Foundation for Biomedical Innovation Research and Innovation at Kobe (FBRI, President: Dr. Tasuku Honjo) is pleased to announce that Dr. Akihiko Taguchi (Department of Regenerative Medicine Research, Institute of Biomedical Research and Innovation, FBRI) has identified that stem cell therapy enhances neurogenesis at hippocampus which has a prominent role in short-term memory. This work has been published as an original research article in Frontiers in Aging Neuroscience.
Summary
Recent trends in this research area: The loss of short-term memory is a major symptom of Alzheimer’s disease. Recent studies have shown that the cells responsible for short-term memory are not present in newborn hippocampus neurons (*1).
Major findings of Taguchi’s study: The number of newborn neurons in the hippocampus is drastically reduced in aged mice that exhibited short-term memory deficits. In contrast, a marked increase in newborn neurons in the hippocampus was observed in aged mice whose short-term memory was improved by stem cell therapy (Figure).
Significance of this study: Previous research targeting the inhibition of the cell death of existing neurons has been conducted extensively worldwide, but has yet to yield conclusive results. The findings of this study led to the identification of a therapeutic target to treat Alzheimer’s disease by promotion of new neuron expression in the hippocampus, such that short-term memory impairment in the diseases can be improved following treatment.
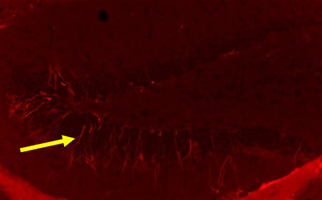
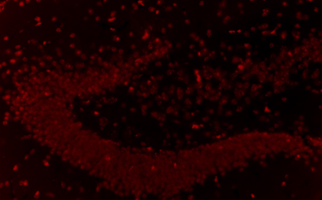
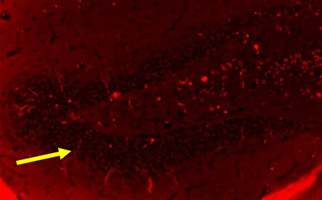
Newborn neurons in the hippocampus of the brain (examples of newborn neurons are indicated by arrows). A large number of newborn neurons are observed in young mice, but few are observed in old mice that exhibit short-term memory deficits. In contrast, a significant number of newborn neurons were observed in aged mice whose short-term memory were improved by stem cell therapy.
Research Background
(1) Necessity for changing the research strategy for Alzheimer’s disease therapy development
Until now, the development of therapies for Alzheimer’s disease has been based on the concept of (a) inhibiting and removing toxic substances such as amyloid-β, (b) suppressing existing neuronal cell death, and (c) preventing the onset of Alzheimer’s disease. However, clinical trials using various therapies have shown unsatisfactory results. Therefore, Taguchi considered that fundamentally change in the concept for treating Alzheimer’s disease is required.
(2) Newborn neurons in the hippocampus are a potential therapeutic target for Alzheimer’s disease
Recent studies have shown that the cells responsible for short-term memory, which are absent in Alzheimer’s disease, are newborn neurons in the hippocampus. These cells are responsible for long-term memory, which are preserved in Alzheimer’s disease. Therefore, Taguchi considered that a rational therapeutic target for Alzheimer’s disease is not the existing neurons but rather the newborn neurons in the hippocampus.
(3) The therapeutic mechanism of stem cell therapy is cell-cell interaction via gap junction (*2).
It is known that cell-cell interaction via gap junction is important for short-term memory in hippocampal newborn neurons. Since Taguchi has discovered and reported that the mechanism of action of hematopoietic and mesenchymal stem cells therapies, which are widely used in regenerative medicine, involves cell-cell interaction via gap junction, Taguchi considered that stem cell therapy may be useful in activating hippocampal newborn neurons.
(4) The mechanism of aging is unknown
The mechanism of aging is still largely unclear, despite the extensive research studies that have been performed. Taguchi discovered and reported that regeneration resulting from stem cell therapies, which is essentially the opposite of aging, is caused by cell-cell interaction via gap junction. Therefore, Taguchi considered that aging and cell-cell interaction via gap junction are likely to be related.
Results
(1) Metabolic state of peripheral blood leukocyte alters with aging
Metabolism-related gene expression in peripheral blood leukocyte cells is altered with age in aged mice.
(2) Stem cells changes the metabolic state of peripheral blood leukocyte via Gap Junction
Intravenous administration of stem cells into aging mice changed the metabolic status of peripheral blood leukocytes of young mice through cell-cell interactions mediated by gap junction.
(3) Cell-cell interaction via gap junction decreases with aging
RNA expression of gap junction is decreased in hippocampal tissues & peripheral blood leukocytes in aged mice and cultured vascular endothelial cells even following passage.
(4) Gap junction are increased by stem cells
RNA expression of gap junction in cultured vascular endothelial cells was increased when co-cultured with hematopoietic stem cells.
(5) Hippocampal newborn neurons drastically decrease with aging
The number of newborn neurons in the hippocampus of aged mice that exhibit short-term memory deficits is drastically reduced.
(6) Stem cells increase the number of newborn neurons in the hippocampus
Intravenous administration of stem cells into aged mice, whose short-term memory were improved by stem cell therapy, significantly increased the number of newborn neurons in the hippocampus.
The significance of this study
(1) Research and development for Alzheimer’s disease:
Our results have revealed that (a) the rational target for Alzheimer’s disease is the activation of newborn neurons in the hippocampus, (b) the development of stem cell therapy offers the potential to cure dementia rather than prevent it, and (c) the central mechanism of its efficacy is cell-cell interaction via gap junction. It is becoming evident that Alzheimer’s disease can be treated from a completely new perspective.
(2) Research for aging
In contrast to many unicellular organisms, the concept of a life span applies to multicellular organisms which is dependent upon numerous cellular processes. Cell-cell interactions via gap junction play an important and direct role in multicellular organisms and this function declines with age. Stem cell-based regeneration enhances these interactions, suggesting that cell-cell interactions via gap junction have a key role during the aging of multicellular organisms. We believe that cell-cell interactions via gap junction will shed a new light on the process of aging science and the development of therapies.
Research Paper
| Authors: | Yukiko Takeuchi1, Orie Saino1, Yuka Okinaka1, Yuko Ogawa1, Rie Akamatsu1, Akie Kikuchi-Taura1, Yosky Kataoka2, 3, Mitsuyo Maeda2, 3, Sheraz Gul4, 5, Carsten Claussen4, 5, Johannes Boltze1, 6, Akihiko Taguchi1 |
|---|---|
| Affiliations: |
1 Department of Regenerative Medicine Research, Foundation for Biomedical Research and Innovation at Kobe, Hyogo, Japan. 2 Multi-modal Microstructure Analysis Unit, RIKEN-JEOL Collaboration Center, RIKEN, Hyogo, Japan. 3 Laboratory for Cellular Function Imaging, RIKEN Center for Biosystems Dynamics Research, RIKEN, Hyogo, Japan. 4 Fraunhofer Institute for Translational Medicine and Pharmacology ITMP, Hamburg, Germany. 5 Fraunhofer Cluster of Excellence for Immune-Mediated Diseases CIMD, Hamburg, Germany. 6 School of Life Sciences, University of Warwick, Coventry, UK. |
| Title of original research article | Increased RNA transcription of energy source transporters in circulating white blood cells of aged mice. |
| Journal: | Frontiers in Aging Neuroscience. |
| DOI: | 10.3389/fnagi.2022.759159 |
Glossary
*1 neurogenesis: Neurogenesis in the hippocampus is known to have a pivotal role in improving memory.
*2 gap junction: Gap junction is an intercellular channel that allows direct diffusion of ions and small molecules, including metabolic substances, between adjacent cells.
Author/Contact
Dr. Akihiko Taguchi

Department of Regenerative Medicine Research,
Institute of Biomedical Research and Innovation,
Foundation for Biomedical Research and Innovation at Kobe
E-mail: taguchi"AT"fbri.org
Please replace “AT” with @ when sending the E-Mail.