Overview
We are developing a drug therapy to regenerate functional neurons without cell transplantation for neurodegenerative diseases, including Alzheimer’s disease. Our first milestone is filing Investigational New Drug applications to FDA and PMDA in 18 months since the US and Japanese are 45.5% of the worldwide dementia drug market. It requires $3 million to achieve this milestone. This innovative technology to increase endogenous stem cells without relying on cell transplantations will be the future of regeneration therapy.
- Technology
- Alzheimer’s disease is a chronic debilitating neurodegenerative disease, essentially “kills” a person long before they are physically dead. It affects millions of people worldwide, is robbing them of memory, humanity, and dignity. With the number of diagnoses on the rise, if we do not find an effective therapy for AD soon, society will lose the ability to support the growing population affected by this disease.
Currently, commercially available drugs for AD are symptomatic therapies. Many pharmaceutical companies tried to develop a cure for AD by focusing on immunotherapy and other treatments to eliminate amyloid-beta (Aβ) depositions. However, to date, all their efforts to prevent plaque formation in the AD brain have failed to benefit the patients.
It has been quite a while since we have shown neural stem cell (NSCs) transplantation improved cognition of aged animals. However, it is challenging to get NSCs for transplantation since they are in the deep part of the brain. We developed a technology to produce pluripotent stem cells from adult mesenchymal stem cells, which allows us to use autologous stem cells for transplantation. Still, it has an issue controlling cell fate and developing tumors. Thus, it seems to take more time for cell transplantation-based neuroregenerative therapy to contribute to medicine than expected.
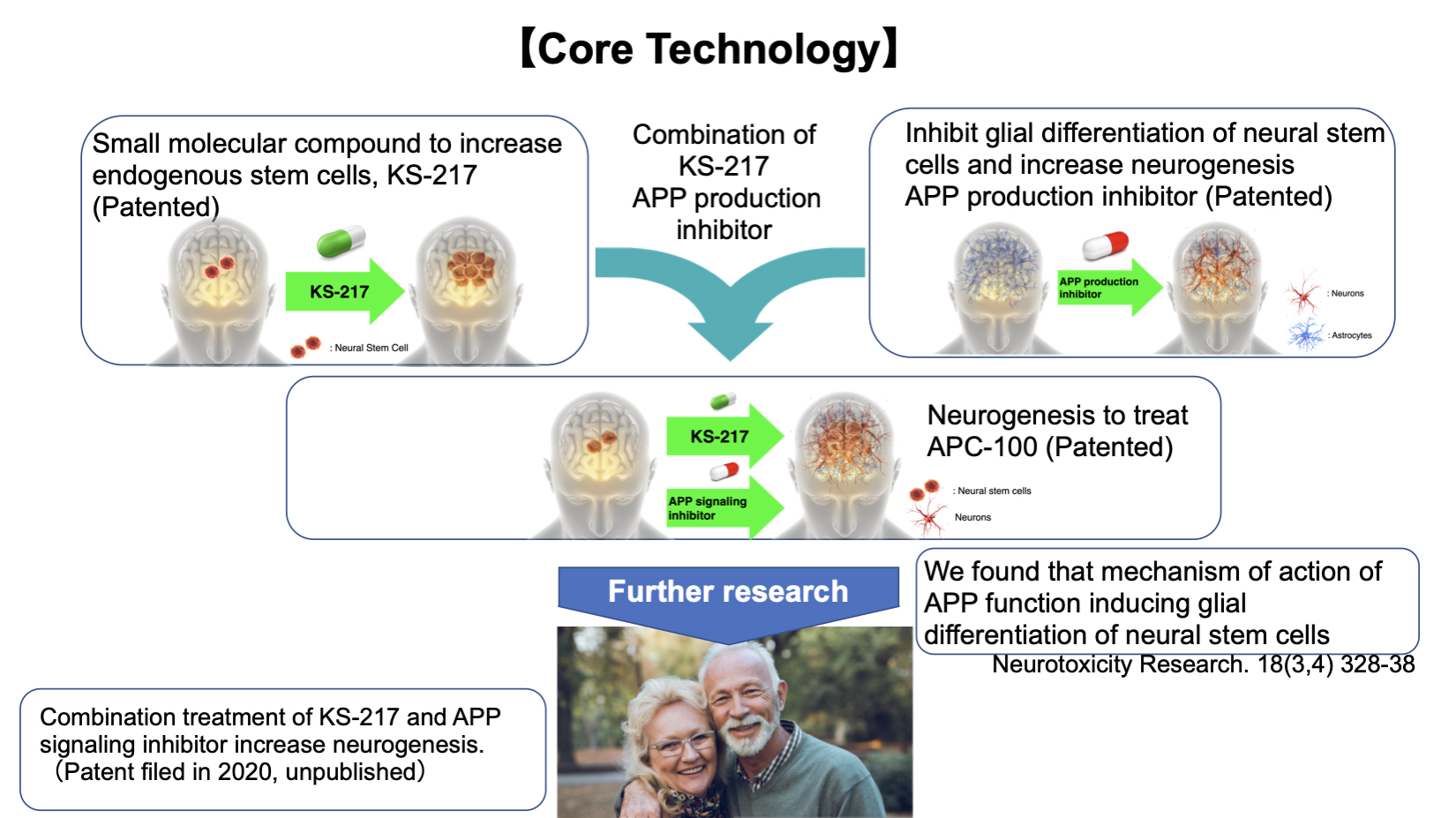
We found a pyropyrimidine derivative that passes through the blood-brain barrier to increase endogenous NSCs significantly. Although atypical antidepressants (SSRIs) have been known to increase neural stem cells, it is up to only 20%. However, our medicine increased NSCs by up to 600% in vivo, eliminating the need for cell transplantation. We also found that the pathological concentration level of amyloid precursor protein (APP) in the AD brain caused glial differentiation of NSCs by activating inflammatory cytokine signaling. We developed combination therapy to modify the AD brain’s inflammatory conditions and increase endogenous NSCs. This treatment proved to increase neurogenesis under AD pathology and reverse the cognitive impairment in model animals.
Our goal is AD therapy, though we would like to develop our technology as an orphan disease therapy to benefit from incentives, including a partial tax credit for clinical trial expenditures, waived user fees, and eligibility for 7 years of marketing exclusivity. Down syndrome (DS) patients have a trisomy of the 21st chromosome, where the APP gene is localized, meaning the patients express excess APP compared to the control subject. Most of the subjects with DS develop early onset of AD. We found that APP induced glial differentiation in NSCs, preventing neurogenesis independent of Aβ production. We have also showed that DS patients’ NSCs behave the same as NSCs overexpressing APP. In some sense, DS subjects are human AD models with overexpression of APP. Thus, we will target developing our technology as an orphan drug for acute regression associated with DS first and then moving to AD therapy.
Further basic pre-clinical efficacy studies, such as in vitro study using iPS cells from DS patients and in vivo study using DS genetic animal models at the University of Central Florida. Pre-clinical safety tests will be conducted by a 3rd party, such as Charles River Laboratories, to deliver independent toxicity and PK/PD studies that the FDA will acknowledge as a credible analysis of the compound. For the Phase 1 clinical study, we will collaborate with UCF, the College of Medicine in the US, and Tohoku University, the school of medicine in Japan, to get human safety and PK/PD results in American and Japanese populations for IND approval by FDA and PMDA. After getting a proof of concept in humans in Phase 2a clinical study, we will seek a pharmaceutical company to license the technology.
Patents- A method of biasing implanted human neural stem cells away from differentiation into glial cells by (+)phenserine to modulate the concentration of soluble βapp in tissue or CSF(US 9,095,573)
- Use of Modified Pyrimidine Compounds to Promote Stem Cell Migration and Proliferation (US 7,687,505, US 8,273,756、US 9,084,789、1/14/2003, 7/21/2015)
- Drug Treatment to Increase Stem Cell Population (CA2473503、EP1471918,603 50 413.2, 1471918FR, JP4623967B, 5414626JPD)
- Combination therapy to improve brain function or promote neurogenesis for treating neurodegenerative conditions (US 62/346,166, PCT/US17/36220)
- Pending
- Combination therapy for neurogenesis (Pending) Filed on 8/27/2020
- Other related patents
- Compositions for Treating or Delaying the Onset of Hair Loss (US 8,791,128, PCT/US09/57134, CA2,736,018, EP2326330, 602009028877.6, 2326330FR, 2324023UK, 5555922JP)
- Vigor Enhancement Via Administration of Pyrimidine Derivatives (CA2,670,341, PCT/US07/85765, EP2324023, 602007047544.9, 2324023FR, 2324023UK, HK1158204B)
- Establishment
- January 24, 2020
- Location
- Kobe International Conference Center S606,6-9-1 Minatojima Nakamachi, Chuo-ward,Kobe-city, Hyogo 650-0046, Japan
- Industry
- Biotech, Drug discovery,
- Indication
- Small molecular compound to increase endogenous stem cells
- Funding Round
- Seed Early
- Raising
- $3million
Team
-
Kiminobu Sugaya, PhD CEO/Co Representative Director
Professor and Head of Neuroscience, Chair of Multidisciplinary Neuroscience Alliance, College of Medicine, University of Central Florida Visiting Professor, Clinical Research, Innovation and Education Center, Tohoku University Hospital Honorary Professor, Instituto de Investigaciones Científicas y Servicios de Alta Tecnología AIP, Panama He has been conducting neuroscience research for more than 30 years in Science University of Tokyo, Southern Illinois University, Mayo Clinic, University of Illinois at Chicago and University of Central Florida.
-
Neung Suh COO/Co Representative Director
Independent investor and consultant for new business development. Experience in working for IT Venture International Marketing PL and a major business company New Business Development Department MG.
She met Dr. Sugaya in the United States, Florida, believed in the potential of his technologies, and decided to develop them together in Japan. -
Bob Hering Director
United States Marine Corps, Colonel, retired
Founder and Former CEO, Con-Air Industries -
Mohtashem Samsam, MD, PhD Director
Director of Research
Advent Health, Research Institute, Orlando, Florida -
David Francis Leon Director
Lawyer, The Florida Bar
Partner
Nelson Mullins Broad and Cassel -
Kyohei Tanaka, CPA Outside director
He is a certified accountant. He led a number of M & A deals such as business integration and acquisition of overseas companies at the investment banking headquarters of a major foreign securities company. Then he moved to a private equity company and engaged in investment business. He established investment hub Co., Ltd. independently in 2018. In 2021, he joined Progenicyte Japan Co., Ltd. As an outside director.