KOBE Biomedical Innovation Cluster newsletter vol. 62
- August 2025 -

vol. 62 August 2025
Hello,
On August 21st, Kobe Biomedical Innovation Cluster (KBIC) welcomed a business delegation centered around Taipei City, Taiwan. On that day, an MOU was signed between Century Biotech Development Company (CBDC) and Foundation for Biomedical Research and Innovation at Kobe (FBRI), with the aim of mutually supporting the commercialization efforts of respective member companies and strengthening collaboration between Taiwan and KBIC. A signing ceremony was held to commemorate this agreement.
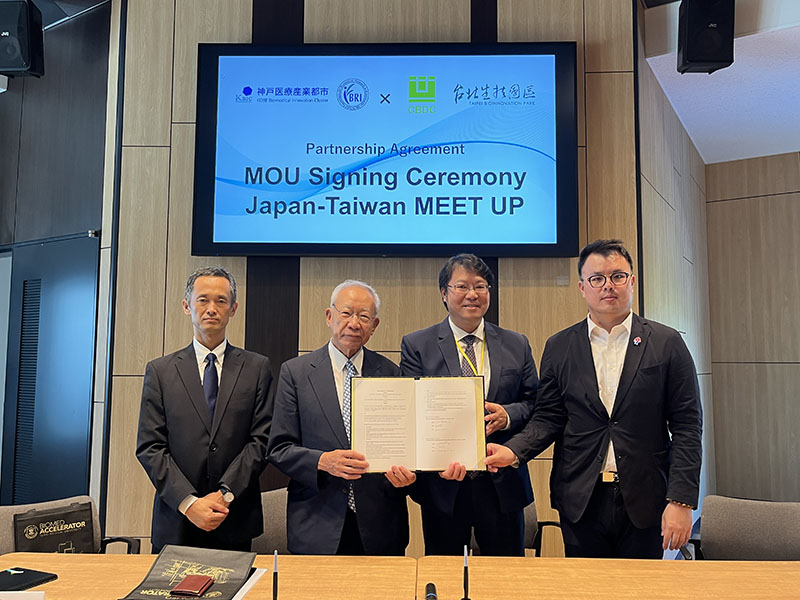
Following the signing ceremony, a networking event was hosted by CBDC and FBRI, where invited Taiwan and KBIC companies gathered. Approximately 50 participants attended the event and each of the companies introduced their businesses, followed by a networking session during which participants exchanged business cards and engaged in active discussion.


Afterward, the group moved to Air Water Inc's International Advanced Medical Center @Kobe, where they were introduced to the company’s products and business operations. The delegation concluded the visit successfully, enriched by a fulfilling experience.

This opportunity served as the kickoff for long-term exchanges between Taiwan and KBIC, and we look forward to strengthening our collaboration and expanding our mutual engagement in the future!
If you missed our past issues of KBIC newsletter, please visit Newsletter Archive.
FBRI Editorial Team
NEWS
Sumitomo Pharma Co., Ltd. and RACTHERA Co., Ltd announcement on the Submission of the Application of Manufacturing and Marketing Authorization for Allogeneic iPS Cell-Derived dopaminergic neural progenitor cells in Japan
Sumitomo Pharma Co., Ltd. (Head Office: Osaka, Japan; “Sumitomo Pharma”) and RACTHERA Co., Ltd. (Head Office: Chuo-ku, Tokyo, Japan;, “RACTHERA”) hereby announce that on August 5, 2025, they have submitted an application of manufacturing and marketing authorization for allogeneic iPS cell-derived dopaminergic neural progenitor cells (INN: “raguneprocel”, “the Product”) for indication of the improvement of motor functions during the off-time period of patients with advanced Parkinson’s disease. The applicant for the authorization is Sumitomo Pharma.
Visit KBIC Website!
We help international medical business thrive in Japan with world-class research institutes and facilities.





 Newsletter Archive
Newsletter Archive